Applications
Cobalt is used in many alloys (superalloys for parts in gas turbine aircraft engines, corrosion resistant alloys, high-speed steels, cemented carbides), in magnets and magnetic recording media, as catalysts for the petroleum and chemical industries, as drying agents for paints and inks. Cobalt blue is an important part of artists’ palette and is used by craft workers in porcelain, pottery, stained glass, tiles and enamel jewelry. The radioactive isotopes, cobalt-60, is used in medical treatment. It is also used to irradiate food, in order to preserve the food and protect the consumer.
Cobalt in the environment
Most of the Earth’s cobalt is in its core. Cobalt is of relatively low abundance in the Earth’s crust and in natural waters, from which it is precipitated as the highly insoluble cobalt sulfine CoS.
Although the average level of cobalt in soils is 8 ppm, there are soils with as little as 0.1 ppm and others with as much as 70 ppm. In the marine environment cobalt is needed by blue-green algae (cyanobacteria) and other nitrogen fixing organisms.
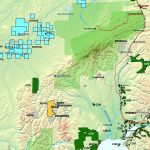
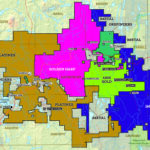
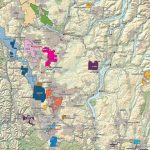
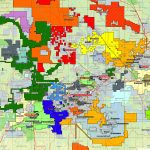
Cobalt is not found as a free metal and is generally found in the form of ores. Cobalt is usually not mined alone. It tends to be produced as a by-product of nickel and copper mining activities. The main ores of cobalt are cobaltite, erythrite, glaucodot, and skutterudite.
The world’s major producers of cobalt are the Democratic Republic of the Congo, mainland China, Zambia, Russia and Australia. It is also found in Finland, Azerbaijan, and Kazakhstan.
World production is 17.000 tonnes per year.
[tv-chart symbol=”TSXV:FCC” width=”980″ height=”610″ language=”en” interval=”D” timezone=”America/Phoenix” theme=”White” style=”1″ toolbar_bg=”#f1f3f6″ enable_publishing=”1″ hide_top_toolbar=”0″ withdateranges=”1″ hide_side_toolbar=”0″ allow_symbol_change=”” save_image=”1″ details=”” hotlist=”” calendar=”1″ stocktwits=”” headlines=”1″ hideideas=”0″ hideideasbutton=”0″ referral_id=””]