Applications
The principal use of silver is as a precious metal and its halide salts, especially silver nitrate, are also widely used in photography. The major outlets are photography, the electrical and electronic industries and for domestic uses as cutlery, jewellery and mirrors.
Both colour and black and white images have relied on silver since the early days of photography: siver bromide and silver iodide are sensitive to light. When light strikes a film coated with one of these compounds, some of the silver ions revert to the metal in tiny nuclei and the film is developed with a reducing agent which causes more silver to deposit on these nuclei. When the negative has the desired intensity, the uneffected silver bromide or iodide is removed by dissoving in a fixing agent, leaving the image behind.
Silver is also employed in the electrical industry: printed circuits are made using silver paints, and computer keyboards use silver electrical contacts.
Silver’s catalytic properties make it ideal for use as a catalyst in oxidation reactions. Other applications are in dentistry and in high-capacity zinc long-life batteries.
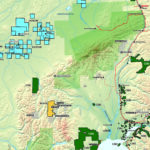
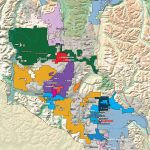
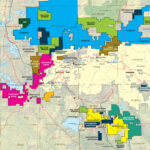
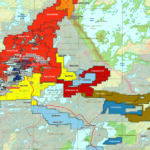
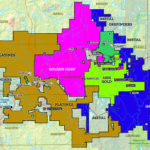
Canadian Silver Production, by region, 2017

Silver in the environment
Silver levels in soil are not usually high except in mineral-rich areas when they can sometimes be as much as 44 ppm. Plants can absorb silver and measured levels come in the range 0.03-0.5 ppm.
Metallic silver occurs naturally as crystals, but more generally as a compact mass; there are small deposits in Norway, Germany and Mexico. The chief silver ores are acanthite mined in Mexico, Bolivia and Honduras, and stephanite, mined in Canada. However silver is mostly obtained as a byproduct in the refining of other metals.
World production of newly mined silver is around 17.000 tonnes per year, of which only about a quarter comes from silver mines. The rest is a byproduct of refining other metals.